SEMINARS 2024
OUR SPEAKERS 2024
- Aaron Mitchell, M.D.
- Adriane Fugh-Berman, MD
- Andrew Reynolds PhD
- Annette Crosse
- Antonio Tito Fojo, MD
- Bhuvan Majmudar
- Carl Elliott, MD, PhD
- Charles L Bennett, MD, PhD
- David Crosby, PhD
- Deborah Cohen
- Frank E. Harrell, Jr. PhD
- George Perry, PhD
- Jack Scannell, PhD
- Joel Lexchin, MD
- Joel S Perlmutter, MD
- John H. Powers, MD
- John Lazo, PhD
- Jonathan Kimmelman, PhD
- Julian Adams, PhD
- Julie M. Eggington, MS, PhD
- Katie Spencer, PhD
- Kim Witczak
- Leeza Osipenko, PhD
- Margaret McCartney, MD
- Marty Tenenbaum, PhD
- Matt Fagg
- Nancy F. Olivieri, MD
- Nick Woolley
- Rudy Tanzi, PhD
- Samra Turajlic MD PhD
- Sharon A. Brangman, MD
- Sir John Burn, PhD
- Steve Wedge PhD
Join vibrant discussions at Consilium Seminars: every other Thursday at 5pm London time
All our public seminars are available on our YouTube channel
SEMINARS 2024
Cancer bioscience: too focused on drug discovery?
January 18, 2024 5pm GMT
Ian Tannock, MD, PhD (Chair)
Discussants:
Richard Sullivan, MD, PhD
Samra Turajlic MD PhD
Andrew Reynolds PhD
Steve Wedge PhD
Jack Scannell, DPhil
«The current privileged focus on biopharmaceuticals is not a long-term, cost-effective approach to cancer control policy, unless it is complemented with public health strategies for cancer prevention»
This statement, published in a recent Lancet Oncology Commission paper, will be discussed by a panel with expertise in cancer policy, basic cancer bioscience and the treatment of cancer, strategies in the pharmaceutical industry for both prevention and treatment, and expertise in the analysis of the economics of the drug industry. Are biopharmaceuticals a long-term approach to reduce cancer mortality? Is basic bioscience research too strongly focused on supporting therapeutic strategies to reduce post-diagnosis cancer mortality? How can the basic bioscience community and the pharmaceutical industry compliment public health strategies for early detection and cancer prevention?
Money Matters: Pharmaceutical R&D for Drugs for Neglected and Orphan Diseases
1 February, 2024 5pm GMT
Joel Lexchin, MD
Neglected diseases affect about 1.9 billion people world-wide compared to orphan diseases which affect about 350 million people. At the same time 10 time more money is spent on research and development for the latter compared to the former. This talk explores these issues and the reason for the disparity.
Imprecision Medicine: The Hidden Errors in Clinical Genetics and Genomics
15 February, 2024 5pm GMT
Julie M. Eggington, MS, PhD
Clinical genetics and genomics is a cornerstone of precision medicine, operating under the principle that a person's inborn genetics or acquired mutations can guide physicians to administer the right drug, at the right dose, at the right time to individual patients. However, too often clinical labs get the DNA sequencing of patients wrong, or report sequencing results with interpretations that are not sufficiently evidence based. Inaccurate genetic and genomic test results can lead to misguided care pathways that are harmful to patients. Such imprecision medicine flourishes in the USA because of the gaps in the U.S.'s patchwork of regulatory oversight. The talk will summarize what is known about the frequency of such errors and the gaps in regulatory oversight that allows errors to persist.
How Pharma Invents Diseases.
February 29, 2024 5pm GMT
Pharmaceutical Companies influence not only what physicians and patients believe about drugs, but also what they believe about diseases. Through third parties that include key opinion leaders (physician influencers), medical societies, and patient groups, pharmaceutical companies have invented diseases and medicalized normal human variations and normal life transitions.
Modernizing Clinical Trial Design and Analysis to Improve Efficiency & Flexibility
March 14, 2024 5pm GMT
Frank Harrell, PhD
This talk covers several ways to make clinical trials more efficient and to reduce the chance of ending with an equivocal result. Some of the approaches covered are Bayesian sequential designs allowing for study extension if results are promising, not being tied by type I assertion probabilities/α spending, using high-information longitudinal ordinal outcomes, and covariate adjustment.
Conflicts of interest in healthcare: where are we now?
March 28, 2024 5pm UK
Conflicts of interest in medicine have been a problem for decades but what are we doing about it? What evidence do we have that it is making any difference? Are conflicts of interest in medicine destined to be a 'forever wicked' problem? This talk is unlikely to give any helpful answers but will try to define the problems that we (still) need answers to.
Conflict-of-interest in oncology: prevalence, trends, and potential harms
April 11, 2024 5pm GMT
Aaron Mitchell, M.D
Despite ongoing concerns and well-publicized scandals, financial conflict-of-interest between US physicians and the drug industry remains common. US physicians receive over $2 billion in personal (non-research) payments from the drug industry annually. Medical oncologists are one of the highest earning specialist groups, especially influential "opinion leaders" who often conduct clinical trials and write clinical practice guidelines. This talk presents and summarizes findings regarding the distribution of industry money across oncologists, cancer hospitals, and - potentially of greatest concern - clinical practice guideline-writing panels. The presentation also shows recent and emerging evidence regarding the potential negative impact of industry money on clinical care delivery.
The Spider Web of Influence: America’s Broken Drug Safety System
April 25, 2024 5pm BST
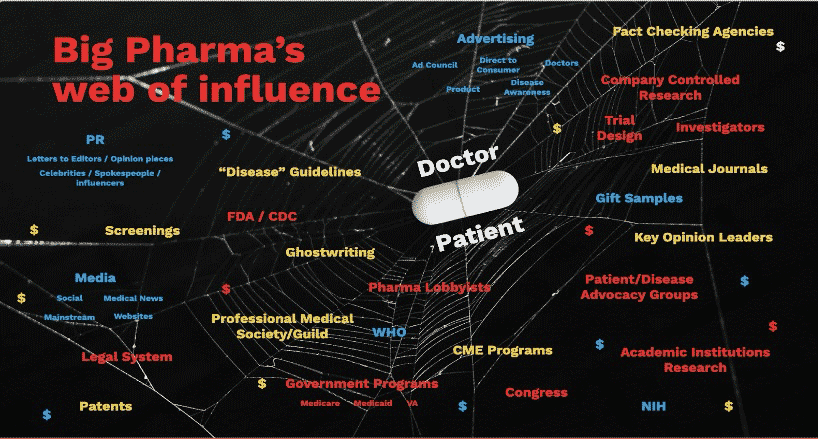
This talk explores the journey of an accidental advocate thrust into the heart of America’s broken drug safety system following her husband’s tragic suicide, despite no history of depression. As a seasoned marketing and advertising professional, Kim Witczak uncovers systemic flaws, shedding light on industry and regulatory priorities, and exposes the intricate web of influence driven by marketing agendas and profit motives, often unbeknownst to doctors and patients alike.
Taking on Big Pharma: Dr Charles Bennett’s Battle
May 16, 2024 5pm BST
Charles L Bennett MD PhD MPP
Stephen Rosen, MD, former provost of the City of Hope, praised Bennett, stating, "Between 1994 and 2010, while at Northwestern’s Medical School, Charlie saved more lives than anyone in American medicine." However, Bennett's efforts have not gone without consequences. The Department of Justice and relevant pharmaceutical corporations responded to his findings by spreading lies about him, resulting in the loss of his position at Northwestern. Bennett was forced to pay $500,000 in a legal settlement, incurring an additional $1 million in legal fees. Former Northwestern University President Mort Schapiro even went as far as to denounce Bennett online and discourage other medical centers from employing him.
The full extent of Northwestern's and the Department of Justice's wrongdoing is detailed in "Taking on Big Pharma: Dr. Charles Bennett's Battle" (published by Skyhorse), which delves into the events surrounding Bennett's battle against the pharmaceutical industry.
Drug development for cancer prevention: opportunities and challenges
May 30, 2024 5pm BST
Andrew Reynolds, PhD
Despite some notable successes, there are still relatively few medicines approved for cancer prevention in healthy individuals. Here we review progress thus far in the development of medicines for cancer prevention and summarise the challenges faced in this space. Furthermore, we outline some key concepts that could further enable or accelerate drug development for cancer prevention in the future. These are summarized under six key themes: (i) unmet clinical need, (ii) patient identification, (iii) risk stratification, (iv) pharmacological intervention, (v) clinical trials, and (vi) health care policy. These concepts, if successfully realised, may help to increase the number of medicines available for cancer prevention.
Unlocking Diversity & Inclusion in Life Sciences
13 June, 2024 5pm BST
This talk from Egality.health focuses on exploring the following issues:
• Why we need diversity in clinical and health services research (including historical practices and current health inequalities)
• Why the life sciences sector needs to change and how
• How to improve communications about research to improve recruitment into clinical trials
• How to engage underserved communities in clinical and health services research
Speakers:
Medical Experimentation and the Price of Saying No
June 27, 2024 5pm BST
Carl Elliott, MD, PhD
In popular culture, whistleblowers are conscience-driven heroes who triumph against the odds. Yet when research abuses occur, medical institutions deny wrongdoing even when it is glaringly obvious, and rarely do mistreated research subjects or their families get any real justice. Whistleblowing is the exception, not the rule. In many scandals, doctors and other staff members remain silent for years while unwitting research subjects are abused. If abuses do come to light, the researchers are usually protected by the medical establishment while the whistleblowers are punished.
Rare degenerative diseases and statistics: Methods for analyzing composite patient outcomes
11 July 2024 5pm BST
In this talk I explain why statistical power is maximized by analyzing the rawest form of clinical trial outcome data, as opposed to analyzing patients at a single time point or reducing rich longitudinal data to time-to-event outcomes. Analyzing all the data longitudinally also provides a formal way to handle missing or partially missing data. A highly flexible longitudinal model will be described in non-mathematical terms. This model is a longitudinal proportional odds logistic model for binary, ordinal, or continuous outcomes, with within-patient serial correlation handled through a simple Markov process in which the patient's previous visit outcome level becomes a predictor for the outcome in the current time period. This is a discrete time state transition model, also called a multistate model, but extended to have an unlimited number of outcome states as long as they can be ordered.
The model may be fitted with standard software, and special Bayesian modeling software has been written for it.
After the model is fitted, a simple multiplication process converts the transition probabilities into current state probabilities ("state occupancy probabilities") to form an intent-to-treat effect that reduces to cumulative incidence of an outcome event if the outcome is binary and terminal. The approach accommodates longitudinal outcome scales with clinical event overrides. The primary clinical readout of this longitudinal ordinal model is the average time in condition y or worse, as a function of time and treatment, for any or all outcome levels y.
This approach has the following as special cases:
- Wilcoxon test for comparing two groups on a single-time ordinal or continuous outcome
- Cox proportional hazards model for comparing time-to-event when the event is terminal
- Parametric longitudinal analysis of continuous outcomes
- Recurrent event analysis
- Joint analysis of recurrent nonfatal events and a terminal event
- Estimation of restricted mean survival time when the event is not necessarily terminal
The model also provides the only formal analysis I know for quantifying evidence that a treatment effects different components of the outcome variable differently. For example, one can test whether a treatment lowers mortality by the same relative amount as it lowers disability. The model is particularly well suited for rare degenerative diseases because of formal handling of death, and because its maximum use of information lowers the sample size needed to achieve adequate power, particularly when there are several follow-up visits.
The method will be briefly compared with time-to-event analysis, DOOR (desirability of outcome ranking), WIN ratio/odds, and time-savings.
A detailed case study of longitudinal ordinal modeling with complete R code may be found at hbiostat.org/rmsc/markov.
Whistleblowing and Whistleblowers in Medical Research: Thirty Years in the Trenches
October 3, 2024 5pm BST
A Conversation with Dr. Nancy Olivieri
Dr. Olivieri will recount her story, and the lessons she has learned during the 30 years of one of the most serious and ongoing ethical scandals in medical history. The conflict began after Dr. Olivieri raised concerns about patient safety in a clinical trial in Toronto. The story moved to villages in Asia, to the European Court of Justice, to a dramatic showdown at the US FDA, and back to more coverups in Toronto. As of 2024, bullying, defamation, firings and endless lawsuits continue, with patients being the most recent victims. Continuing long after the unsolved death of Barry Sherman (CEO of Apotex, the drug company at the center of the scandal), this is a story of coverups: of false and misleading science, of research and professional misconduct (some immortalized by John le Carré in the Constant Gardener), of regulatory capture and of the complicit silence of nearly everyone in power in Canadian academia.
Inside Healthcare Investigations: Exposing the Realities of Clinical Research
October 31, 2024, 5pm London UK
Join Dr. Deborah Cohen, an award-winning investigative journalist and broadcaster, for an insightful conversation on uncovering critical issues in healthcare and clinical research. With her extensive experience in leading major investigations for The BMJ, BBC Newsnight, Panorama, and Channel 4 Dispatches, Dr. Cohen has exposed systemic challenges, driven policy change, and held powerful institutions accountable. In this session, she will share her approach to combining rigorous data analysis with storytelling, and discuss how investigative journalism can illuminate critical health and research issues that impact patients, practitioners, and policymakers alike. This is a unique opportunity to hear from a trailblazer who has transformed the way complex healthcare topics are communicated to the public and the global stage.
Do cancer patients benefit by participating in clinical trials?
December 5, 2024 5pm London UK time
Many oncologists, patients, cancer centers, and cancer professional societies regard participation in a cancer trial as a therapeutic option. These claims are often based, ironically, on anecdote and observational studies - precisely the kind of evidence clinical trials aim at superseding. In this talk, I present a series of philosophical and empirical analyses of the therapeutic value of trial participation. I close by discussing some of the limitations of my analyses, and unpacking some of its ethical and policy implications.

